St. Philip Neri Club Fundraises for the Cystic Fibrosis Foundation
March 6, 2019
Throughout March, the St. Philip Neri Club will be promoting a fundraiser for the Cystic Fibrosis Foundation, an organization that helps to fund CF research and helps CF families pay for treatment. Cystic Fibrosis is a genetic disease affecting the secretory glands. These glands are responsible for multiple biological processes, like sweat secretion and mucus production. CF patients produce a thicker, stickier mucus than healthy individuals which affects the respiratory, digestive, and reproductive systems. An estimated 30,000 Americans live with the disease, and every year another 1,000 individuals are diagnosed with it. Cystic Fibrosis patients endure a daily onslaught of symptoms and treatments. These individuals are fighters of a daily battle.
My cousin Angela is one such fighter. Diagnosed at birth, Angela has spent 9 years with CF. During the day, Angela has to go through a regiment of treatments to stay healthy. She begins by taking albuterol, a drug designed to open up her airways. Angela then uses a device called Vest Therapy. This device generates vibrations to help shake up and clear the mucus in her lungs.

The Vest Therapy is used alongside two additional treatments. First, Angela uses the Vest with sodium chloride for 10 minutes in order to loosen the mucus. Then, she uses the Vest for another 10 minutes with a drug called Pulmozyme. This is a very expensive drug that is a DNA breakdown of Angela’s mucus. Lastly, Angela inhales Flovent, a steroid.
However, Angela’s treatments don’t stop there. Every time she eats a fatty food, she has to take two creon pills. Cystic Fibrosis patients do not generate sufficient enzymes to break down fatty substances. Creon gives patients the necessary enzymes to break down fats for usage in the body.

In addition to all these treatments, Angela started a new drug in October of 2017, called Orkambi. Orkambi is supposed to help the cells in Angela’s body. Since Angela began this treatment, she has had better respiratory function and has gain considerable weight, which is very important for CF patients.
This past October, however, Angela stopped taking Orkambi and began a clinical trial for Symdeko. Symdeko has already been approved by the FDA for ages 12 and up. Now, the drug is being tested on younger children. Symdeko is similar to Orkambi but should be more effective.
The Symdeko study lasted from October 2018 to December 2018. Within the study, no patients were given placebos; two different formulas were distributed to the participants. The company that ran the clinical trial will review the data collected over the winter. In the spring, the trial will be reopened to the participants using the formula with better results. From there, Symdeko will hopefully be approved by the FDA for younger patients.
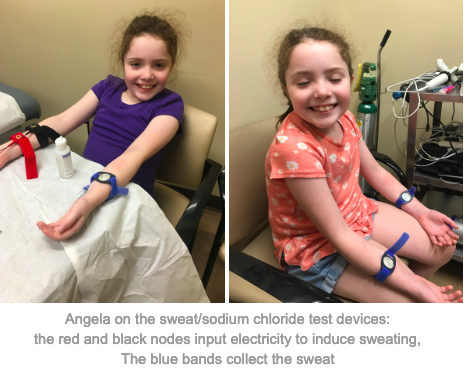
Angela has done well with Symdeko. She says that she has fewer stomach issues on the drug. In addition, her sodium chloride levels have become normal, and she has performed better on breathing tests. This is incredibly exciting for the CF community. These results show that Symdeko is working to correct the underlying causes of the disease.
Living with CF is undoubtedly a challenge. People like Angela endure a daily fight, and their courage is admirable. Thankfully, we are winning this fight. Breakthroughs, like Symdeko, are occurring more frequently. The life expectancy for CF patients has skyrocketed over the past few decades; climbing from childhood to 37 and still growing. However, the fight isn’t over. There is more work to be done, more long days of treatments, and more breakthroughs that will come. With your help, these breakthroughs can come faster. If you are interested in making a donation to the Cystic Fibrosis Foundation, contact me at [email protected] Thank you for your support!

